NHS gets 12 years to roll out new weight-loss drug Mounjaro
 Getty Images
Getty ImagesA new weight-loss vaccine is set to be rolled out in the UK but it could take 12 years before everyone has a shot, the NHS medicines advisory body has said.
The National Institute for Health and Care Excellence’s (NICE) final draft guidance on Mounjaro is recommended to be available from March, along with advice on diet and exercise.
It will be available to people – potentially 3.4 million people – who have a body mass index (BMI) over 35 and have at least one obesity-related health problem.
But amid concerns it could overwhelm services, particularly GPs, NICE agreed to give the NHS more than a decade to introduce it – an unprecedented move for the drug.
NICE chief medical officer Professor Jonathan Benge admitted this meant “many people will have to wait”.
But he said: “We must make this difficult decision to protect vital NHS services and test ways to deliver a new generation of weight loss drugs.”
Patient groups have expressed disappointment at the decision to give the NHS so long.
The problem still exists
Initially only those patients receiving specialist weight management services will have access to the service – matching the approach taken by similar weight loss drug Wegovy.
But from June the NHS will start offering the service to others.
It’s unclear exactly how this will be done – GPs are likely to be responsible for referring patients, but the question remains who will provide ongoing support including diet, exercise and monitoring.
NHS England is expected to publish guidance in the new year. It may involve using an app or setting up a separate service to support GPs.
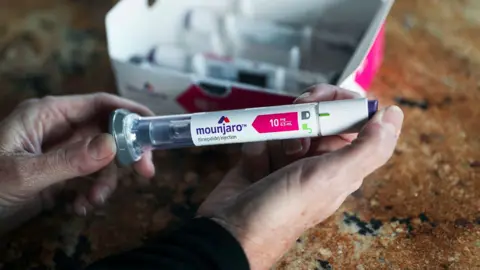
Mounjaro (also known as tezepatide), made by Eli Lilly and Company, can make you feel fuller so you eat less. In trials, people wearing it lost a fifth of their weight.
 The Washington Post via Getty Images
The Washington Post via Getty ImagesWegovy, also known as semaglutide, is already used on the NHS and works in a similar way. But this is only available to those receiving professional weight loss management.
It is estimated that around 40,000 people are in this position.
These medications can be purchased privately. Both drugs are also already available to patients with type 2 diabetes – although semaglutide is marketed to these patients under the name Ozempic.
The launch of Mounjaro provides the public struggling with severe obesity with a next-generation weight loss pill option.
To be eligible, patients also need to have an obesity-related disease, such as diabetes, high blood pressure or heart disease.
Under the rollout plan, patients with the highest clinical need will be prioritized first.
About 250,000 people are expected to benefit in the first three years.
NICE then plans to evaluate how it works before moving forward with a wider rollout.
As this is the final draft guidance, the rollout could still be delayed if anyone appeals the decision. If not, the guidance will be stamped before Christmas, NICE said.
worry
Mounjaro, a weekly injection, costs the NHS £122 per patient per month, but NICE considers it cost-effective given the costs of obesity.
Once use is discontinued, users may gain weight back.
Welsh ministers will use guidance from NICE to guide its rollout.
It has been recommended for use in Scotland – although the NHS is reportedly struggling to promote it.
Helen Kirrane of Diabetes UK said Mounjaro could play an “important” role in tackling obesity.
But she added: “We are concerned about how long it might take for people to gain access.”
Dr Kath McCullough, NHS England’s national specialist adviser on obesity, said weight loss pills were an “important tool” to help tackle “one of the biggest public health issues facing the NHS” “.
She said the phased rollout was needed to protect patients’ access to other NHS services they rely on.
Professor Camilla Hawthorne, of the Royal College of GPs, warned the drug should not be seen as a “magic bullet”, adding that it was not without risks and was not the right treatment for everyone who was eligible. plan.




